EXPANDING OUR STAR
The star of innovation, Hyundai Sungwoo is
moving towards a new future in the business of
auto parts industry.
WAY OVER the LIMIT
Surpassing challenges and limits,
our battery business is constantly setting
new standards in the battery industry.
PIONEERING SPIRIT
We secure continuous growth engines
through innovative technology and quality
management.
-
COMPANY
-
PRODUCT
-
R&D
-
CUSTOMER SUPPORT
-

COMPANY

We introduce Hyundai Sungwoo Solite, a company striving to reach beyond the limits and set the standard for the battery industry. -

PRODUCT

With unrivaled quality and technologcal competitiveness, we bring customer satisfaction across various segments of the global battery industry. -

R&D
We prepare for the future battery industry with advanced technological innovation. -

CUSTOMER SUPPORT
Hyundai Sungwoo Solite will do its best to deliver customer satisfaction with infinite dedication.
- Company
- Vision & Philosophy
- History
- Network
-

-

-
Corporate philosophy rooted in respect for humanity. Mission to satisfy the needs of customers. With our vision and philosophy, we will further enrich lives and enhance technologies.

-

-

-
Our pioneering spirit to venture out and create new paths is the foundation of Hyundai Sungwoo. In the next 10 years, we will envision and practice technology that changes the paradigm of the global market.

-

-

-
Firmly rooted in the spirit of innovation and challenge, Hyundai Sungwoo pursue growth and expansion.

-

We keep challenging towards
a better future for mankind.
The history of Hyundai Sungwoo is
the footprint of the Korean auto parts technology.
Growing as a leading company that
sets new global standards based on
technology innovation.
- Product
- Automotive
- Leisure Battery
We set the standard for
automotive batteries with ideal
product design and quality.
Solite has become the standard of good batteries.
We develop and produce automotive batteries
of the highest quality and stability.
We provide powerful and durable
battery while enjoying
exciting outdoor activity.
Solite Batteries for recreational vehicle(RV) meet various demands from engine starting
to
deep cycling service for electric application. These dual purpose batteries are the
ideal
compromise between high starting and extra reserve power for accessory loads.
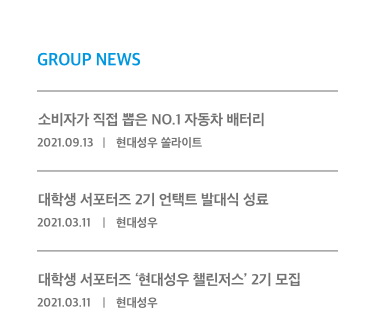
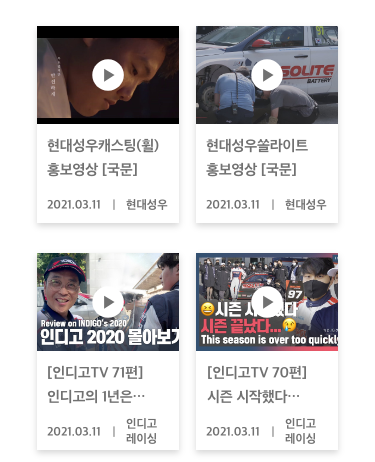
- PR Center
- PR Material
- PR Films